bromine orbital notation|Electron Configuration Chart of All Elements (Full Chart) : Tagatay In this catalysis, 2.2 equivalent of water was necessary to obtain a high amide yield because water promotes triboroxine hydrolysis. Based on the X-ray structure of a complex of methylboronic acid, phenylpruvic acid, and 1,2,3,4-tetrahydroquinoline, a cyclic active . At the latest definition: . See examples of AT THE LATEST used in a sentence.
PH0 · Organoboron catalysis for direct amide/peptide bond formation
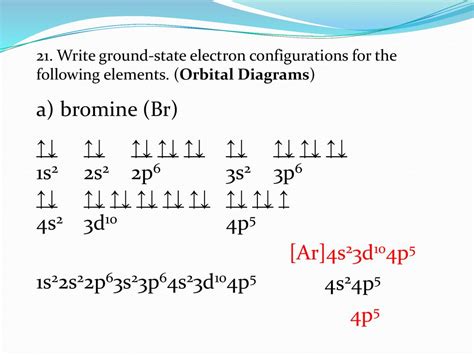
PH1 · How to Write the Atomic Orbital Diagram for Bromine (Br)
PH2 · Electron Configuration Chart of All Elements (Full Chart)
PH3 · Electron Configuration
PH4 · Complete Electron Configuration for Bromine (Br, Br
PH5 · Bromine orbital diagram
PH6 · Bromine Electron Configuration (Br) with Orbital Diagram
PH7 · Bromine
PH8 · Atomic Data for Bromine (Br)
PH9 · 2.4 Electron Configurations
This highlights how damn good Witcher 3’s side quests are. Read Next: The 10 Funniest Moments In Witcher 3. Gleb Oleinik. Gleb has played PC games since the late 1990s and has always enjoyed RPGs the most. He had tons of fun playing Witcher 3 and learning about the greater Witcher universe, so he made this website for news, .
bromine orbital notation*******In this catalysis, 2.2 equivalent of water was necessary to obtain a high amide yield because water promotes triboroxine hydrolysis. Based on the X-ray structure of a complex of methylboronic acid, phenylpruvic acid, and 1,2,3,4-tetrahydroquinoline, a cyclic active . To write the orbital diagram for the Bromine atom (Br) first we need to write the electron configuration for just Br. To do that we need to find the number o.March 23, 2023 Jay. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the .
Bromine Orbital Diagram. In this article today we are going to tell you about the electron configuration of Bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Please read the full .
Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content To understand this principle, let's consider the bromine atom. Bromine (Z=35), which has 35 electrons, can be found in Period 4, Group VII of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will .The individual orbitals are represented, but the spins on the electrons are not; opposite spins are assumed. When representing the configuration of an atom with half filled orbitals, indicate the two half filled orbitals. The expanded notation .Br I Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 0 4s 2 4p 5 2 P° 3 / 2 Ionization energy 95284.8 cm-1 (11.8138 eV) Ref. T63 Br II Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 0 4s 2 4p 4 3 P 2 .
To illustrate the bromine orbital diagram, begin by determining the number of electrons from the periodic table. Take note of the electron configuration for reference and .Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal .
Bromine electron configuration notation. The electronic configuration notation of Br is represented by the notation; Br : Ar 18 4s 2 3d 10 4p 5. Bromine unabbreviated electron configuration. Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. When there is no noble gas configuration for the starting electrons, this is referred to as an unabbreviated .
In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura.
Orbital Energies and Atomic Structure. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \(l\) differ so that the energy of the orbitals increases within a shell in the order s < p < d < f.
Orbital diagrams are a common way of showing electron configurations in which the orbitals are shown as boxes and the electrons as arrows. Skip to content. Chemistry Steps. . What is the ground-state electron configuration of bromine (Br)? 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10 4p 5 [Kr] 4s 2 3s 10 4p 5 [Ne] 3s 2 3p 5 [Ar] 4s 2 3d 10 4p 5; 1s 2 .Figure 2.5.6 The Three Equivalent 2p Orbitals of the Hydrogen AtomThe surfaces shown enclose 90% of the total electron probability for the 2p x, 2p y, and 2p z orbitals. Each orbital is oriented along the axis indicated by the subscript and a nodal plane that is perpendicular to that axis bisects each 2p orbital.
Electron Configuration Chart of All Elements (Full Chart) The noble gas configuration for bromine is : [Ar]3d^(10)4s^(2)4p^(5) The previous noble gas is argon which has the electron configuration of : 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6) We call this the argon core. It's a handy way of writing out electron structures without writing all the inner electron shells. The angular momentum quantum number, #l#, which represents different subshells can be used to find the maximum number of electrons in the subshell, and the number of orbitals using #2(2l+1)# and #2l+1#, respectively. An #l# of 0 corresponds to the #s# subshell, 1 corresponds to the #p# subshell, 2 to the #d# subshell, and 3 to the #f# subshell. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. . Bromine (Br) 36: Krypton (Kr) 37: Rubidium (Rb) 38: Strontium (Sr) 39: Yttrium (Y) 40: Zirconium (Zr) 41: Niobium (Nb) 42: Molybdenum (Mo) 43 .
The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic numbers.. Here is a look at how to .
Filling Orbital: 4p 5; Number of Electrons (with no charge): 35; Number of Neutrons (most common/stable nuclide): 45; Number of Protons: 35; Oxidation States: ±1,5; . Bromine - Br (EnvironmentalChemistry.com)- Comprehensive information for the element Bromine - Br is provided by this page including scores of properties, element names in .

Electronic configuration of the Bromine atom. Valence electrons. Orbital diagram. Bromine electron configuration. ← Electronic configurations of elements . Br . Electronic configuration of the Bromine atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5.bromine orbital notation Electron Configuration Chart of All Elements (Full Chart)Electronic configuration of the Bromine atom. Valence electrons. Orbital diagram. Bromine electron configuration. ← Electronic configurations of elements . Br . Electronic configuration of the Bromine atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5.d orbitals, and two from the n value for the f orbitals. This periodic table has the electron configuration for each row written along the left hand side using the method just outlined. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons.
Bromine orbital diagram Solved show the orbital-filling diagram for br (bromine). Orbital bromine diagram electron filling. Bromine Orbital Diagram. Copy of electron configuration, orbital notation and quantum numbers Bromine orbital diagram filling br show sulfur orbitals diagrams 2s2 energy rule wiringall homonuclear diatomic bonding 2p What .bromine orbital notationBromine (Br) element properties, information, facts, uses and Periodic Table trends. Complete information about the Bromine element - Atomic Number 35, atomic mass [79.904], melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties .Give the electron configuration for carbon in complete spdf notation, in noble gas abbreviated notation, and in orbital diagram notation. Define orbital diagram. Write the full electron configuration, the orbital box diagram, and the noble gas shorthand configuration for .Bromine (Br) has an atomic mass of 35. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic Composition; 67 Br: 67: 66.96479(54)# 68 Br: 68: 67.95852(38)# 69 Br: 69: 68.95011(11)# Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting .
Worlds Hardest Game 3 unblocked - Clasroom 6x, Classroom6x.Github.io: Play instantly in fullscreen browser, no downloads, no ads. Explore and enjoy various gaming experiences now! . In World's Hardest Game 3, you control a red square navigating through a maze of balls to move between rooms. Use the arrow keys to maneuver .
bromine orbital notation|Electron Configuration Chart of All Elements (Full Chart)